Personal collections


Oxygen in the gaseous state appears in the form of a molecule with two oxygen atoms 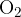 . The molar mass of an oxygen molecule in kilogram is 32 kg. Calculate the number of oxygen atoms in 10 kg of oxygen and take Avogadro constant as
. The molar mass of an oxygen molecule in kilogram is 32 kg. Calculate the number of oxygen atoms in 10 kg of oxygen and take Avogadro constant as  .
.