Personal collections


The nuclide notation of the isotope strontium-90 is 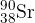 . Which statement is correct?
. Which statement is correct?
A nucleus of strontium-90 has 38 neutrons.
A nucleus of strontium-90 has 52 neutrons.
A nucleus of strontium-90 has 90 electrons.
A nucleus of strontium-90 has 90 neutrons.