Personal collections


Atoms consist of (see Figure 1):
Nucleus. The nucleus consists of:
protons - these are particles that have a positive electric charge;
neutrons - these are particles that do not have an electric charge and are therefore electrically neutral.
Electron shell. The electron shell is the space in which electrons are located, which are very small, light, and negatively charged particles. Electrons are also responsible for bonding atoms into molecules and matter.
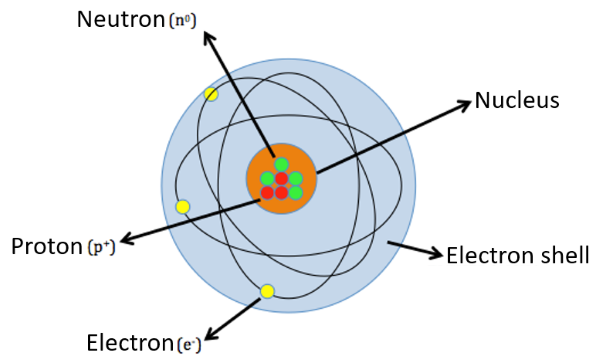
Figure 1: A sketch of the structure of an atom. An electron cloud is not necessarily spherical but electrons can move in different spatial arrangements called electron shells or orbitals
Electrons are bound to the nucleus by the electric force of attraction between them and protons. In the formation of matter, individual atoms are bound into molecules, crystals, and other configurations, and in this process, electrons, more precisely the electric force between electrons, play a key role, thus determining the properties of all substances that we can see and feel.
Atoms consist of a positively charged nucleus, which contains protons and neutrons, and negatively charged electrons, which make up the outer shell. The arrangement and interactions of electrons determine the chemical properties of atoms, molecules, and crystals.
The atom is also discussed from a slightly more chemical point of view in the material, Particles in an atom.
Atoms differ from each other in the number of electrons, protons, and neutrons that make them up. We denote these numbers as:
Z - number of protons (also called the atomic number);
N - number of neutrons;
A - number of protons and neutrons (also called the mass number).
It therefore implies that:
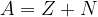
An atom is externally electrically neutral, so the number of electrons is equal to the number of protons.
Atoms are classified in the periodic table according to the number of protons in the nucleus:
in the 1st place is the element with one proton - hydrogen;
in the 2nd place in the periodic table is the element with two protons - helium;
....
in the 8th place is the element with eight protons - oxygen;
....
in the 79th place is the element with 79 protons - gold;
etc ...
Precisely, because the number of protons determines the order of the elements in the periodic table of elements, the number of protons is also called the ordinal number. Atoms that have the same number of protons are said to belong to the same element.
Since the masses of atoms are very small, a useful unit for calculation is the atomic unit of mass, denoted by 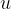 , which is equal to 1/12 of the mass of the carbon isotope
, which is equal to 1/12 of the mass of the carbon isotope 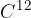 . Its value is:
. Its value is:
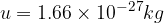
The masses of the proton and neutron are very similar and are very close to the atomic mass unit 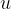 , while the mass of the electron is much smaller:
, while the mass of the electron is much smaller:
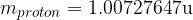
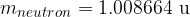
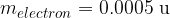
When comparing the masses, we can see that almost all the mass of the atom is collected in the nucleus (masses of electrons are negligible). When calculating the mass of an atom, we will so often assume that it is equal to the sum of the masses of protons and neutrons in the nucleus (electrons will not be taken into account).
The size of an atom determines the size of the electron shells, i.e. the space through which the electrons move. This space is not precisely defined, but we can say that the average radius of an atom is 100 picometres, so:
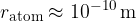
The nucleus of an atom itself is 100,000 times smaller: the radii of the nuclei are between 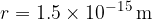 and
and 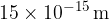 . We notice that - although all the mass of the atom is concentrated in the nucleus - almost the entire volume of the atom is represented by the electron shell.
. We notice that - although all the mass of the atom is concentrated in the nucleus - almost the entire volume of the atom is represented by the electron shell.