Personal collections


Matter is everything that surrounds us. How we observe it depends on whether we observe it as a chemist, physicist, geologist, biologist, etc. A chemist is interested in the structure of matter and studies the laws according to which matter changes. Physicists are interested in its physical properties. Just like chemistry, physics is interested in the structure of matter, the size and mass of molecules and atoms, and the forces between them, as both the physical and chemical properties of matter depend on this.
The physical properties of a substance are density, elasticity, compressibility, strength, thermal and electrical properties, etc. We will learn about density in this material, and all the others in separate materials.
Even when it is (chemically speaking) the same substance, its physical properties can be different. What do they depend on? Let's list some quantities that affect the physical properties of substances:
Temperature
State of a substance
The same substance can appear in three states. These are solid, liquid, and gas. Liquid and gas are also called fluid states, as the substance can flow and take the shape of the container in which it is located. A substance can change from one state to another under the influence of heat. The physical properties of the same substance change dramatically after these transitions.
Pressure
The substance can be homogeneous or inhomogeneous:
Homogeneous substance
In the case of a homogeneous substance, its physical properties are the same everywhere. They are independent of the place within the substance from where we take the sample for measurement.
Inhomogeneous substance
In the case of an inhomogeneous substance, its physical properties depend on the location. Judging whether an object is homogeneous or not depends on the size of the particle within the substance being observed.
A substance is denser if there is a greater mass of the substance in the same volume.
The density 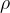 of a substance is therefore the quotient of its mass
of a substance is therefore the quotient of its mass 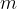 and volume
and volume 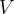 :
:

The unit for density is:
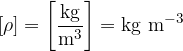
The density 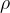 of a substance is given as:
of a substance is given as:
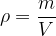
or the mass 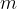 is:
is:
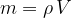
The table of the density of some substances at temperature 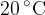 is as shown below:
is as shown below:
Similar to how density is the ratio of mass to volume, specific weight is the ratio of weight 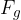 to volume
to volume 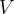 . The symbol of specific weight is
. The symbol of specific weight is 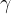 :
:

The unit for specific weight is:
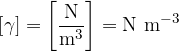
How are density and specific weight related?
Let's write again the formula of the specific weight 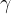 which is given as:
which is given as:
The specific weight 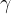 of a substance is therefore its density
of a substance is therefore its density 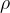 multiplied by the gravitational acceleration
multiplied by the gravitational acceleration 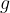 . It is not constant for a certain substance but rather depends on the magnitude of the gravitational acceleration, which varies slightly depending on the place of observation. Therefore, in practice, instead of specific weight
. It is not constant for a certain substance but rather depends on the magnitude of the gravitational acceleration, which varies slightly depending on the place of observation. Therefore, in practice, instead of specific weight 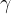 , we prefer to use the product
, we prefer to use the product 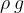 .
.
Let's make 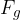 the subject of equation 2:
the subject of equation 2:
Specific weight 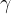 of a substance is given as its weight
of a substance is given as its weight 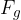 divided by the volume
divided by the volume 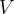 :
:
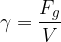
Specific weight 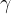 and density
and density 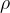 are related through free-fall acceleration, by the equation:
are related through free-fall acceleration, by the equation:
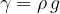
The weight of a substance can also be expressed in terms of density, as: