Personal collections


Define the binding energy of a nucleus.
A stationary nucleus of uranium-238 (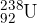 ) decays to form a nucleus of thorium-234 (
) decays to form a nucleus of thorium-234 (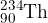 ). An
). An 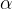 -particle and a gamma-ray photon are emitted. The equation representing the decay is
-particle and a gamma-ray photon are emitted. The equation representing the decay is
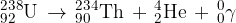
The masses of the nuclei are given in the table below.
State the relationship between the binding energies of the nuclei that is consistent with this reaction being energetically possible.
Calculate, for this reaction
the change, in 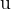 , of the mass,
, of the mass,
the total energy, in 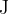 , released.
, released.
State and explain whether the energy of the gamma-ray photon is equal to the energy released in the reaction.