Personal collections


What is the frequency of the photon needed to ionize a hydrogen atom with an electron in the second excited state? In the ground state, the electron of a hydrogen atom has an energy of 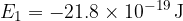 . The speed of light is
. The speed of light is 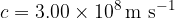 , the Planck constant
, the Planck constant 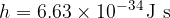 .
.