Personal collections


The figure below shows the visible part of the emission spectrum from hydrogen gas in a laboratory on the Earth. The numbers indicate the wavelength, in 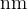 , represented by each line.
, represented by each line.
Explain how the emission spectrum provides evidence for the existence of discrete energy levels for the electron in a hydrogen atom.
The figure below shows five of the energy levels in the hydrogen atom. The wavelengths of radiation shown in the figure above relate to transitions to the 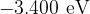 level in the figure below.
level in the figure below.
Show that the energy level X is 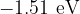 .
.