Personal collections


A beam of light consists of a continuous range of wavelengths from 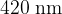 to
to 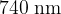 . The light passes through a cloud of cool gas, as shown in Figure I below.
. The light passes through a cloud of cool gas, as shown in Figure I below.
The spectrum of the light emerging from the cloud of cool gas is viewed using a diffraction grating. Explain why this spectrum contains a number of dark lines.
Some of the electron energy levels of the atoms in the cloud of gas are represented in Figure II below.
Light of wavelength 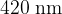 has a photon energy of
has a photon energy of 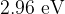 . Calculate the photon energy, in
. Calculate the photon energy, in 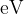 , of light of wavelength
, of light of wavelength 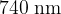 .
.
Use data from (i) and your answer in (i) to show, on figure II, the changes in energy levels giving rise to the dark lines in (a).