Personal collections


State what is meant by a photon.
Light in a beam has a continuous spectrum that lies within the visible region. The photons of light have energies ranging from 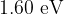 to
to 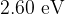 . The beam passes through some hydrogen gas. It then passes through a diffraction grating, and an absorption spectrum is observed.
. The beam passes through some hydrogen gas. It then passes through a diffraction grating, and an absorption spectrum is observed.
All of the light absorbed by the hydrogen is re-emitted. Explain why dark lines are still observed in the absorption spectrum.
Some of the energy levels of an electron in a hydrogen atom are illustrated in the figure below.
The dark lines in the absorption spectrum are the result of electron transitions between energy levels. On the figure, draw arrows to show the initial electron transitions between energy levels that could give rise to dark lines in the absorption spectrum.
Calculate the shortest wavelength of the light in the beam.