Personal collections


State what is meant by a photon.
Describe the appearance of a visible line emission spectrum, as seen using a diffraction grating.
The lowest electron energy levels in an isolated hydrogen atom are shown in the figure below.
An electron is initially at the energy level 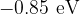 . State the total number of different wavelengths that may be emitted as the electron de-excites (loses energy).
. State the total number of different wavelengths that may be emitted as the electron de-excites (loses energy).
Photons resulting from electron de-excitation from the 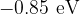 energy level are incident on the surface of a sample of platinum. Platinum has a work function energy of
energy level are incident on the surface of a sample of platinum. Platinum has a work function energy of 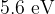 . Determine
. Determine
the maximum kinetic energy, in 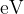 , of a photoelectron emitted from the surface of the platinum,
, of a photoelectron emitted from the surface of the platinum,
the wavelength of the photon producing the photoelectron in (ii) part 1.