Personal collections


A hydrogen atom 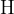 and a once ionized helium atom
and a once ionized helium atom 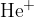 have one outer electron. We knock out this one outer electron in each of them using some energy.
have one outer electron. We knock out this one outer electron in each of them using some energy.
Choose the correct statement about the energy with the correct explanation from the list below:
The energy consumed is the same for hydrogen and helium since in both cases, we emit one electron.
The energy consumed is higher with helium, as it has two more neutrons in the nucleus as opposed to hydrogen, which has none.
The energy consumed is higher with hydrogen as the electron orbits at a greater distance from the nucleus.
The energy consumed is higher in helium because it has more nucleons in the nucleus than hydrogen.
The energy consumed is higher in helium as it has more protons than hydrogen.
The energy consumed is higher with hydrogen as hydrogen is lighter and has higher kinetic energy.