Personal collections


We have a hydrogen atom, with an electron in the ground state at an energy of 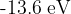 . What does ionization energy mean and how much is it in this case? Choose the correct explanation and the correct value from the list below:
. What does ionization energy mean and how much is it in this case? Choose the correct explanation and the correct value from the list below:
Ionization energy is the electron energy of 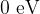 .
.
Ionization energy is the energy of the first excited state and is 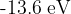 .
.
Ionization energy means the energy lost by the atom in a collision with another atom and amounts to 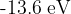 .
.
Ionization energy is the energy required to convert the atom into an ion and is 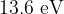 .
.
Ionization energy is the energy of an ion and is 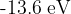 .
.
Ionization energy is the energy of an ion and is 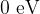 .
.
Ionization energy is the energy required to convert the atom to an isotope and is 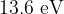 .
.