Personal collections


A 10-liter tire is inflated with air at 17 °C to a pressure of 2.21 bar. Due to the hot summer, it heats up to 45 °C, as does the air in it.
We think that all the molecules that make up air are the same. How many “air molecules” would need to be removed or added to the tire to maintain pressure and volume?
What must be the ratio of the volumes at the beginning and end for the pressure and the number of molecules to be maintained?
What can be the maximum temperature of the asphalt to keep the tire from bursting? The maximum tire pressure can be 2.55 bar. Treat it like a rigid, airtight body.
The general gas constant is 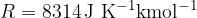
The Avogadro constant is 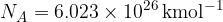 .
.