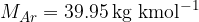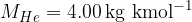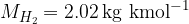Personal collections


In a 10-litre container is a mixture of helium and argon at a temperature of 273 K and a total pressure of 1 bar. The initial partial pressure ratio, defined as: 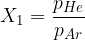 is equal to 1.2. The initial mass of the mixture is 10 g. Then, 2 g of hydrogen gas
is equal to 1.2. The initial mass of the mixture is 10 g. Then, 2 g of hydrogen gas 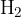 is added to the mixture.
is added to the mixture.
What is the ratio 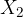 of the partial pressures of helium and argon after the addition of hydrogen?
of the partial pressures of helium and argon after the addition of hydrogen?
What is the ratio 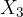 of the partial pressures of helium and hydrogen? Write the result to 3 decimal places.
of the partial pressures of helium and hydrogen? Write the result to 3 decimal places.
Gas constant:
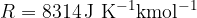
The kilomolar masses of the mentioned elements: