Personal collections


A room has the shape of a square with a volume of 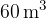 . The pressure and the mass of air in the room are 1.1 bar and 80 kg respectively. The average molar mass of air is
. The pressure and the mass of air in the room are 1.1 bar and 80 kg respectively. The average molar mass of air is 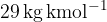 .
.
What is the air temperature in the room?
The room's door is opened for a moment so that the pressure drops to 0.9 bar and the temperature remains the same. How many air molecules escaped from the basement, assuming all the “air molecules” are the same?
Take 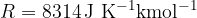 as the gas constant and
as the gas constant and 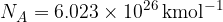 .
.