Personal collections


In a 100 liter container is air at a temperature of 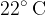 and a pressure of 1010 bar. 2 g of water is poured into the container. The water is allowed to evaporate and the temperature drops to
and a pressure of 1010 bar. 2 g of water is poured into the container. The water is allowed to evaporate and the temperature drops to 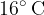 .
.
What is the partial pressure of the water vapour in the tank?
What is the total pressure?
The molar mass of water is 18 kg.