Personal collections


2 kg of water at a temperature of 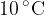 is poured into a thermally insulated container. 0.5 kg of an unknown metal at a temperature of
is poured into a thermally insulated container. 0.5 kg of an unknown metal at a temperature of 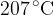 is dropped inside the water in the container. When thermal equilibrium is established, a mixture temperature of
is dropped inside the water in the container. When thermal equilibrium is established, a mixture temperature of 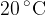 is obtained. What is the specific heat capacity of the metal? What metal is it?
is obtained. What is the specific heat capacity of the metal? What metal is it?
The specific heat of the water is 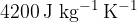 .
.