Personal collections


We place 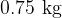 of water at
of water at 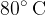 and
and 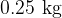 of water at
of water at 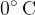 in a thermally insulated container and observe the final temperature at equilibrium to be
in a thermally insulated container and observe the final temperature at equilibrium to be 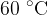 . By how much has the internal energy of the warmer water, colder water, and total internal energy changed?
. By how much has the internal energy of the warmer water, colder water, and total internal energy changed?
The specific heat capacity of water is 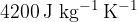 .
.