Personal collections


Select the correct option(s) in each of the list below:
Which of the following can be considered as internal energy?
Kinetic energy of a body.
Kinetic energy of molecules of matter.
Potential energy of a body.
Elastic energy of the molecules of matter.
What is the difference between internal energy and heat?
No differences.
Heat is just a type of internal energy.
Heat is the energy that is transferred between bodies and thus increases or decreases their internal energy.
How does the internal energy depend on the speed of motion of the molecules of matter?
It does not depend.
It increases with the speed of the molecules.
It decreases with the speed of the molecules.
Can the molecules of matter be completely at rest?
Yes, it can be at a temperature of 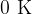 .
.
No, it can't be.
How can we change the internal energy?
By adding or subtracting heat.
By adding or subtracting mechanical work.
By adding or subtracting heat and mechanical work.