Personal collections


A sealed container of fixed volume 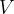 contains
contains 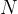 molecules, each of mass
molecules, each of mass 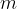 , of an ideal gas at pressure
, of an ideal gas at pressure 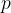 .
.
State an expression, in terms of 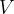 ,
, 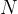 ,
, 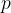 and the Boltzmann constant
and the Boltzmann constant 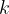 , for the thermodynamic temperature
, for the thermodynamic temperature 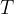 of the gas.
of the gas.
Show that the mean translational kinetic energy 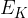 of a molecule of the gas is given by
of a molecule of the gas is given by
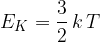
Explain why the internal energy of the gas is equal to the total kinetic energy of the molecules.