Personal collections


A square box of volume 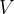 contains
contains 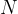 molecules of an ideal gas. Each molecule has mass
molecules of an ideal gas. Each molecule has mass 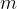 . Using the kinetic theory of ideal gases, it can be shown that, if all the molecules are moving with speed
. Using the kinetic theory of ideal gases, it can be shown that, if all the molecules are moving with speed 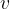 at right angles to one face of the box, the pressure
at right angles to one face of the box, the pressure 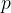 exerted on the face of the box is given by the expression
exerted on the face of the box is given by the expression

This expression leads to the formula

for the pressure 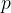 of an ideal gas, where
of an ideal gas, where 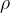 is the density of the gas and
is the density of the gas and 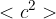 is the mean-square speed of the molecules. Explain how each of the following terms in equation 2 is derived from equation 1:
is the mean-square speed of the molecules. Explain how each of the following terms in equation 2 is derived from equation 1:
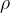 : ...........................................................................
: ...........................................................................
 : ...............................................................
: ...............................................................
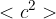 : ......................................................................
: ......................................................................
An ideal gas has volume, pressure and temperature as shown in the figure below.
The mass of the gas is 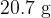 . Calculate the mass of one molecule of the gas.
. Calculate the mass of one molecule of the gas.