Personal collections


In an experiment, 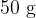 of water at
of water at 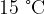 is poured into an insulated beaker.
is poured into an insulated beaker. 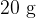 of ice at
of ice at 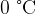 is added to the water. The water is stirred until it's temperature has fallen to
is added to the water. The water is stirred until it's temperature has fallen to 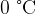 . What will be the specific latent heat of fusion of ice from the experiment if
. What will be the specific latent heat of fusion of ice from the experiment if 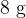 of ice does not melt and the specific heat capacity of water is
of ice does not melt and the specific heat capacity of water is 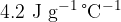 .
.