Personal collections


An ideal gas has a volume of 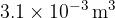 at a pressure of
at a pressure of 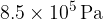 and a temperature of
and a temperature of 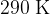 , as shown in the figure below.
, as shown in the figure below.
The gas suddenly expands to a volume of 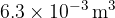 . During the expansion, no thermal energy is transferred. The final pressure of the gas is
. During the expansion, no thermal energy is transferred. The final pressure of the gas is 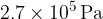 at temperature
at temperature 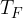 , as shown in the figure above.
, as shown in the figure above.
Show that the number of gas molecules is 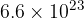 .
.
Show that the final temperature 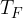 of the gas is
of the gas is 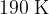 .
.