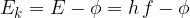Electromagnetic waves are transverse wave of electric and magnetic field. Electromagnetic waves include among others:
visible light,
radio wave,
microwaves,
X-ray radiation.
In certain cases, however, electromagnetic waves appear to consist of particles or "energy packets". These packets are called photons. Through experiments, we found that their energy 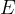 is directly proportional to the frequency
is directly proportional to the frequency 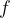 of the electromagnetic wave:
of the electromagnetic wave:
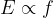
Sometimes an electromagnetic wave cannot be described as a wave but must be treated as if it consists of particles - photons. Photons are not observed in everyday life, they can only be observed when we observe very weak light with a very sensitive light detector.
As already mentioned in the introduction, the photon energy depends linearly on the wave frequency:
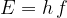
where the proportionality constant 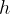 is Planck's constant with the value:
is Planck's constant with the value:
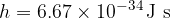
Frequency 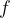 and wavelength
and wavelength 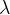 are directly related in electromagnetic waves by the equation:
are directly related in electromagnetic waves by the equation:
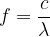
where  is the speed of light. In a vacuum, the speed of light is:
is the speed of light. In a vacuum, the speed of light is:
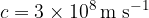
The formula for the photon energy can thus be expressed as:
A photon is the basic particle of an electromagnetic wave. Its energy depends on the wavelength or frequency of light and is related to it by the equation:
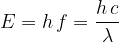
where 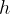 is Planck's constant which is given as:
is Planck's constant which is given as:
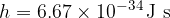
and  is the speed of light.
is the speed of light.
As we can see in the example above, the energies of individual photons are extremely small. This gives us an inkling that the number of photons coming from, for example, the sun must be extremely large, since we feel their energy well as heat.
For calculations with very small energies, such as we are dealing with on the atomic scale, electron volts are used as the unit of energy instead of Joules (for easier calculations). One electron volt is the energy gained by an electron with a base charge 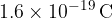 when it crosses a voltage difference of one volt. 1 electronvolt is equal to:
when it crosses a voltage difference of one volt. 1 electronvolt is equal to:
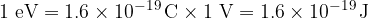
One electron volt (1 eV) is the energy gained by an electron when it crosses a voltage difference of one volt.
The photoeffect or photoelectric phenomenon is a phenomenon in which an electromagnetic wave falls on matter and ejects electrons from it. This is one of the examples that can be explained much more easily by the existence of photons than by electromagnetic waves. Let's look at the scheme of the phenomenon:
If light photons hit a metal (the metal is usually connected to a cloud of free electrons, i.e. a metallic bond), they can knock electrons out of it under certain conditions. In this case, the ejected electron receives all the energy of a certain photon, and the photon itself disappears.
To exit the metal, the electron consumes energy  , which is called work function. The work function is necessary for the electron to overcome the electric attraction of the network of atoms, and the rest of the received energy is converted into the kinetic energy of the now free electron. The kinetic energy of the ejected electron is thus equal to:
, which is called work function. The work function is necessary for the electron to overcome the electric attraction of the network of atoms, and the rest of the received energy is converted into the kinetic energy of the now free electron. The kinetic energy of the ejected electron is thus equal to:
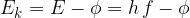
The work function  depends on the type of metal since the electrons in different metals are bound differently strongly. Consequently, if the energy of the photon is less than the work function, then such a photon cannot knock out an electron.
depends on the type of metal since the electrons in different metals are bound differently strongly. Consequently, if the energy of the photon is less than the work function, then such a photon cannot knock out an electron.
The current that flows in an electric circuit, despite the fact that there is no voltage source in it, is called dead current.
Figure 2 shows the dead flow diagram. The circuit consists of a cathode and an anode, which are located in a vacuum-evacuated glass container. The cathode and anode are a connected circuit, but it is broken inside the vacuum vessel. Light falls on the cathode, which knocks electrons out of it due to the photoelectric effect. Some electrons fly in the direction of the anode and reach it. This movement of electrons causes the cathode to become positively charged and the anode to become negatively charged, and an electric current flows between them.
If light photons knock electrons out of a metal, we say that the photoelectric effect is taking place. The kinetic energies of the ejected electrons are given as: