Personal collections


We are used to the chemical elements around us being stable. This means that if we isolate them from the rest of the world, they do not change over time.
But this does not apply to all elements, as some elements are unstable. This means that the elements would change even if they were completely isolated from their surroundings. Their instability is not the result of environmental influences, but rather a property of the elements.
To put it more precisely, the nuclei of these elements are unstable. By this we mean that the nuclei of unstable elements change over time to a stable state, emitting particles or radiation. The process in which nuclei spontaneously emit various particles or radiation is called radioactivity. Radioactivity is the way unstable nuclei with higher energy are converted into stable nuclei with lower energy.
The following radioactive decays of nuclei are known:
Alpha and beta decay
In both decays, the number of protons in the nucleus is reduced, and the original element is transformed into a new element. When the nucleus decays, it emits particles or radiation.
Gamma decay
In gamma decay, the nucleus does not reduce due to the reduction in the number of protons but rather the reduction in the:
internal energy state or
number of neutrons.
When the nucleus decays, it emits particles or radiation, and the element can - in the case of a change in the number of neutrons - change from one isotope to another.
The decay process of individual nuclei is completely random. Therefore, the time when a particular nucleus will decay cannot be predicted. What we can predict, however, is how fast a set of (identical) nuclei will decay. Namely, each radioactive substance has its own decay rate. We most often use the half-life as a measure of this speed.
The half-life is the time it takes for half of all the initial nuclei to decay. It is denoted by 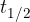 .
.

Figure 1: Decay of radioactive nuclei as a function of time. After the half-life  , the mass of the nucleus is reduced to half
, the mass of the nucleus is reduced to half
The half-life is that key piece of information, with the help of which we can calculate the decrease in the number  of nuclei with time. When calculating, we use the following equation:
of nuclei with time. When calculating, we use the following equation:
The decay rate of radioactive nuclei is calculated using the formula:
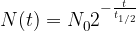
where:
 is the initial number of all nuclei at the beginning of the decay measurement;
is the initial number of all nuclei at the beginning of the decay measurement;
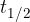 is the half-life, i.e., the time in which one-half of the initial nuclei decays.
is the half-life, i.e., the time in which one-half of the initial nuclei decays.
Sometimes, when calculating the decay of nuclei, we use a transformed equation in which the decay constant  appears instead of the half-life
appears instead of the half-life  . The equation is:
. The equation is:
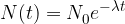
where  and
and 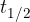 are related by a factor of the natural logarithm of 2:
are related by a factor of the natural logarithm of 2:
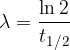
The three most common and first discovered types of radioactive decay are:
alpha decay (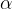 ),
),
beta decay (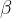 ),
),
gamma decay (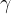 ).
).
Let’s take a closer look at them.
In alpha decay, the initial nucleus decays into:
an alpha particle which consists of two protons and two neutrons, and
the final nucleus, which is reduced by two protons and two neutrons.
An atomic nucleus moves to a lower energy state when it decays. Nuclear decay also creates "excess energy", which is released as:
the kinetic energy of the alpha particle and
the kinetic energy of the nucleus.
However, since the nucleus is usually much heavier than the alpha particle, most of the kinetic energy is received by the alpha particle.
As mentioned, by emitting an alpha particle, the nucleus loses two protons and two neutrons. Therefore:
the atomic number 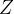 of the nucleus reduces by 2 while
of the nucleus reduces by 2 while
its mass number 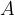 reduces by 4.
reduces by 4.
The initial nucleus is therefore transformed into a new element.
In beta decay, the initial nucleus decays into:
electron,
neutrino, and
the final nucleus in which one neutron changes into a proton.
Since a proton is created in the nucleus from a neutron, the nucleus - to maintain the total charge - emits a negative beta particle, which is an electron. In addition, the decay produces a particle with no mass and no charge, which is called a neutrino and denoted as 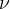 .
.
In doing so,:
the mass number 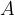 is therefore preserved, and
is therefore preserved, and
the atomic number 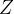 increases by 1.
increases by 1.
The reaction can be written as:
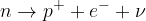
The energy released in the decay is mostly converted into the kinetic energy of the electron and neutrino.
A gamma-active nucleus emits its excess energy by emitting a gamma photon. In doing so, the composition of the nucleus does not change.
The activity of a substance is determined by the number of decays per second. The greater the activity:
the greater the amount of substance and/or
the higher the rate of nuclear decay.
The activity is denoted by  and calculated according to the formula:
and calculated according to the formula:
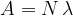
where lambda ( ) is the decay constant and
) is the decay constant and 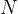 is the number of decaying nuclei. The unit for activity is:
is the number of decaying nuclei. The unit for activity is:
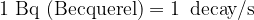
As an interesting point, let us add that the old unit for activity was defined as the activity of one gram of radium 226. It was called Curie. The old and new units are connected by the equation:
