Personal collections


Hydrogen and deuterium can be represented by the nuclide symbols 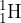 and
and 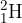 , respectively. What is a difference between hydrogen and deuterium?
, respectively. What is a difference between hydrogen and deuterium?
The deuterium atom has twice the number of electrons as the hydrogen atom.
The deuterium nucleus has a charge, but the hydrogen nucleus has no charge.
The deuterium nucleus has less mass than the hydrogen nucleus.
The deuterium nucleus has half the charge per unit mass of the hydrogen nucleus.