Personal collections


Radon-222 is a radioactive element having a half-life of 3.82 days. Radon-222, when found in atmospheric air, can present a health hazard. Safety measures should be taken when the activity of radon-222 exceeds 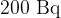 per cubic metre of air.
per cubic metre of air.
Define radioactive decay constant.
Show that the decay constant of radon-222 is 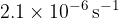 .
.
A volume of 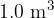 of atmospheric air contains
of atmospheric air contains 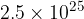 molecules. Calculate the ratio
molecules. Calculate the ratio
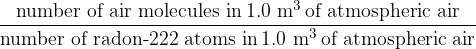
for the minimum activity of radon-222 at which safety measures should be taken.