Personal collections


Copper-66 is a radioactive isotope. When a nucleus of copper-66 decays, the emissions include a 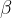 −particle and a
−particle and a 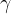 -ray photon. The count rate produced from a sample of the isotope copper-66 is measured using a detector and counter, as illustrated in the figure below.
-ray photon. The count rate produced from a sample of the isotope copper-66 is measured using a detector and counter, as illustrated in the figure below.
State three reasons why the activity of the sample of copper-66 is not equal to the measured count rate.
In a time of 42.0 minutes, the count rate from the sample of copper-66 is found to decrease from 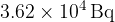 to
to 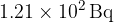 . Calculate the half-life of copper-66.
. Calculate the half-life of copper-66.
The 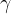 -ray photons emitted from radioactive nuclei have specific energies, dependent on the nucleus emitting the photons. By comparison with emission line spectra, suggest what can be deduced about energy levels in nuclei.
-ray photons emitted from radioactive nuclei have specific energies, dependent on the nucleus emitting the photons. By comparison with emission line spectra, suggest what can be deduced about energy levels in nuclei.