Personal collections


A sample of a radioactive isotope contains 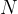 nuclei of the isotope at time
nuclei of the isotope at time 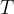 . At time (
. At time (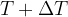 ), the sample contains (
), the sample contains (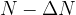 ) nuclei of the isotope. The time interval
) nuclei of the isotope. The time interval 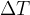 is short. Use the symbols
is short. Use the symbols 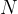 ,
, 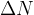 ,
, 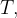 and
and 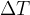 to give expressions for:
to give expressions for:
the average activity of the sample during the time 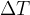 ,
,
the probability of decay of a nucleus in the time 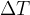 ,
,
the decay constant 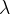 of the isotope.
of the isotope.
The isotope polonium-208 (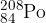 ) is radioactive and decays to form lead-204 (
) is radioactive and decays to form lead-204 (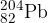 ). The nuclear equation for this decay is
). The nuclear equation for this decay is
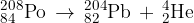
Data for nuclear masses are given in the table below.
Determine, for the decay of one nucleus of polonium-208:
the change, in 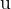 , of the mass
, of the mass
the total energy, in 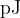 , released.
, released.
The polonium-208 nucleus is initially stationary. The initial kinetic energy of the 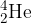 nucleus (
nucleus (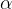 -particle) is found to be less than the energy calculated in (i) part 2. Suggest two possible reasons for this difference.
-particle) is found to be less than the energy calculated in (i) part 2. Suggest two possible reasons for this difference.