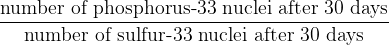Personal collections


The isotope phosphorus-33 (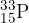 ) undergoes
) undergoes 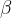 -decay to form sulfur-33 (
-decay to form sulfur-33 (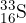 ), which is stable. The half-life of phosphorus-33 is 24.8 days.
), which is stable. The half-life of phosphorus-33 is 24.8 days.
Define radioactive half-life.
Show that the decay constant of phosphorus-33 is 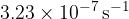 .
.
A pure sample of phosphorus-33 has an initial activity of 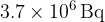 . Calculate
. Calculate
the initial number of phosphorus-33 nuclei in the sample,
the number of phosphorus-33 nuclei remaining in the sample after 30 days.
After 30 days, the sample in (b) will contain phosphorus-33 and sulfur-33 nuclei. Use your answers in (b) to calculate the ratio