Personal collections


The thermal equilibrium of a substance is a state when all particles of a substance have the same temperature or the same kinetic energy (for more knowledge on the heat and kinetic energy of the particles of a substance, see the material, Heat and Temperature). Another condition for a state of equilibrium is also that there is no external source of heat or heat losses. This is achieved in a thermally insulated container called a calorimeter. It is the characteristic of a calorimeter that during the measurement, the heat losses are negligible.
We must distinguish the equilibrium state from the steady state.
Equilibrium is established in a heat-insulated container - a calorimeter, in which we put e.g. two substances at different temperatures. The warmer substance emits the same amount of heat as the cooler substance receives. After a certain time, all the particles of the substance have the same temperature, which is called the final temperature of the mixture.
In a steady state, the observed substance is not thermally insulated, but heat continuously flows into the substance and the same heat also leaves - see Heat conduction. An example of a steady state is a constant temperature in a closed, heated room in winter. The heat emitted by the stove during the observed time is the same as the heat that passes through the walls to the outside air, which is at a lower temperature. Even in this case, all air molecules in the interior can have the same temperature, which is a result of the equality of incoming and outgoing heat.
Let's consider two substances with different specific heat capacities, masses, and temperatures. We put them in a heat-insulated container, which is called a calorimeter, and wait until thermal equilibrium is established. Let's assume that the aggregate state - the state of matter (solid, liquid, gas) will not change during the establishment of thermal equilibrium.
The substance that is at a higher temperature (see Figure 1):
has temperature 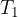 , mass
, mass 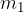 and specific heat capacity
and specific heat capacity 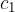 , and
, and
emits heat to the substance at a lower temperature and then cools down.
The substance that is at a lower temperature (see Figure 1):
has temperature 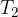 , mass
, mass 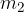 and specific heat capacity
and specific heat capacity 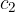 , and
, and
receives heat and then heats up.
Heat passes from the warmer substance to the cooler one until thermal equilibrium is established. Then all the particles of matter are at the same temperature. Let's call it the final mixture temperature 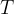 .
.
The internal energy does not change since there is no external heat and external work:
A calorimeter is a thermally insulated container. If several substances are placed in the calorimeter at different temperatures, thermal equilibrium is established after a certain time - particles of all substances are at the same temperature.
Since there is no external source of heat or work, the law of conservation of energy applies:
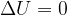
The heat lost by the warmer body is equal to the heat gained by the colder body:
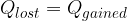
We speak of change in physical state when a substance changes from a solid to a liquid state, from a liquid to a gaseous state, or vice versa when heated (or cooled).
One key characteristic of the process of a change in physical state is that the substance remains at the same temperature until it completely changes to another state. This temperature is called the melting point and the boiling point.
The heat quantity 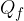 needed to completely melt a substance of mass
needed to completely melt a substance of mass 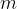 in a solid state, which is already at the melting point temperature, is calculated using the formula:
in a solid state, which is already at the melting point temperature, is calculated using the formula:
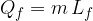
where 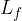 is called the specific latent heat of fusion.
is called the specific latent heat of fusion.
The heat quantity 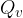 required to completely evaporate a substance of mass
required to completely evaporate a substance of mass 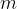 in the liquid state, which is already at the boiling point, is calculated using the formula:
in the liquid state, which is already at the boiling point, is calculated using the formula:
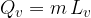
where 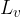 is called the specific latent heat of vaporization.
is called the specific latent heat of vaporization.
Substances in different physical states and at different temperatures can be placed in the calorimeter. During the establishment of an equilibrium state, a substance can pass from one state to another. Even in this case, it is considered that a substance that is at a higher temperature gives off heat to a substance at a lower temperature. The heat given off is equal to the heat received:
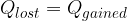
When receiving or emitting heat, a substance can pass from one phase state to another. In the tasks under this chapter, in addition to the question about the final mixture temperature, it is also possible to ask what state the substance is in after thermal equilibrium is established.
We can tackle the task by:
first looking at how much heat the warm body would give off if it cooled down to a selected temperature, e.g. 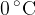 , and
, and
then we look at how much heat we need to heat the cold body to the same temperature.
If the difference is positive:
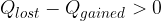
then the mixture of the substances is reheated to the final mixture temperature with residual heat.
If the difference is negative,
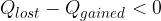
then we need to take the heat away from the total mass again and look at what we get.