Personal collections


2 kg of ice at a temperature of 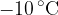 and 2 kg of steam at a temperature of
and 2 kg of steam at a temperature of 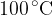 are placed in a thermally insulated container. What happens to the mixture when thermal equilibrium is established?
are placed in a thermally insulated container. What happens to the mixture when thermal equilibrium is established?
The specific heat capacity of ice is 2090 J/kg K and that of water is 4200 J/kg K. The specific latent heat of fusion of ice is 334 kJ/kg and the specific latent heat of vaporisation of water is 2.26 MJ/kg.