Personal collections


Define specific heat capacity.
A student carries out an experiment to determine the specific heat capacity of a liquid using the apparatus illustrated in the figure below.
Liquid enters the tube at a constant temperature of 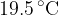 and leaves the tube at a temperature of
and leaves the tube at a temperature of 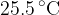 . The mass of liquid flowing through the tube per unit time is
. The mass of liquid flowing through the tube per unit time is 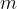 . Electrical power
. Electrical power 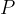 is dissipated in the heating coil. The student changes
is dissipated in the heating coil. The student changes 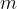 and adjusts
and adjusts 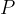 until the final temperature of the liquid leaving the tube is
until the final temperature of the liquid leaving the tube is 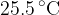 . The data shown in the table below are obtained.
. The data shown in the table below are obtained.
Suggest why the student obtains data for two values of 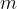 , rather than for one value.
, rather than for one value.
Calculate the specific heat capacity of the liquid.