Personal collections


Define specific latent heat of fusion.
A mass of 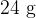 of ice at
of ice at 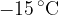 is taken from a freezer and placed in a beaker containing
is taken from a freezer and placed in a beaker containing 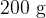 of water at
of water at 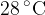 . Data for ice and for water are given in the figure below.
. Data for ice and for water are given in the figure below.
Calculate the quantity of thermal energy required to convert the ice at 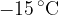 to water at
to water at 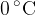 .
.
Assuming that the beaker has negligible mass, calculate the final temperature of the water in the beaker.