Personal collections


A polystyrene cup contains a mass of 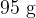 of water at
of water at 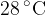 . A cube of ice of mass
. A cube of ice of mass 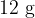 is put into the water. Initially, the ice is at
is put into the water. Initially, the ice is at 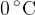 . The water, of specific heat capacity
. The water, of specific heat capacity 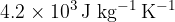 , is stirred until all the ice melts. Assuming that the cup has negligible mass and that there is no heat exchange with the atmosphere, calculate the final temperature of the water. The specific latent heat of fusion of ice is
, is stirred until all the ice melts. Assuming that the cup has negligible mass and that there is no heat exchange with the atmosphere, calculate the final temperature of the water. The specific latent heat of fusion of ice is 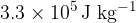 .
.