Personal collections


A beaker contains a liquid of mass 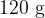 . The liquid is supplied with thermal energy at a rate of
. The liquid is supplied with thermal energy at a rate of 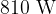 . The beaker has a mass of
. The beaker has a mass of 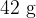 and a specific heat capacity of
and a specific heat capacity of 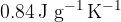 . The beaker and the liquid are in thermal equilibrium with each other at all times and are insulated from the surroundings. The figure below shows the variation with time
. The beaker and the liquid are in thermal equilibrium with each other at all times and are insulated from the surroundings. The figure below shows the variation with time 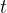 of the temperature of the liquid.
of the temperature of the liquid.
Determine the specific heat capacity, in 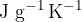 , of the liquid.
, of the liquid.