Personal collections


An aluminium can of mass 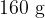 contains a mass of
contains a mass of 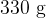 of warm water at a temperature of
of warm water at a temperature of 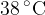 , as illustrated in the figure below.
, as illustrated in the figure below.
A mass of 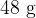 of ice at
of ice at 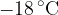 is taken from a freezer and put into the water. The ice melts and the final temperature of the can and its contents is
is taken from a freezer and put into the water. The ice melts and the final temperature of the can and its contents is 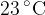 . Data for the specific heat capacity
. Data for the specific heat capacity  of aluminium, ice, and water are given in the table below.
of aluminium, ice, and water are given in the table below.
Assuming no exchange of thermal energy with the surroundings,
show that the loss in thermal energy of the can and the warm water is 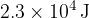 ,
,
use the information in (a) to calculate a value 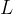 for the specific latent heat of fusion of ice.
for the specific latent heat of fusion of ice.