Personal collections


A liter of water at a temperature of 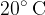 is placed in a thermally insulated container together with an electric immersion heater with a power of 1 kW. The heater is on for 30 minutes. What happens to the water?
is placed in a thermally insulated container together with an electric immersion heater with a power of 1 kW. The heater is on for 30 minutes. What happens to the water?
The specific heat capacity of water is 4200 J/kg K and the specific latent heat of vaporization of water is 2260 kJ/kg.