Personal collections


Matter consists of basic particles - atoms. Atoms are rarely free in nature, so they are usually combined into larger groups - molecules. Molecules can consist of atoms of the same substance or of different substances. The attractive forces that bind atoms to molecules are always electrical in nature.
Atoms are arranged in the periodic table according to the number of electrons orbiting the nucleus. In the first place is hydrogen with one electron. The number of electrons in an atom determines the element's serial number (ordinal number), and their arrangement in energy levels determines the properties of the element.
There is the same number of protons in the nucleus as there are electrons. Electrons and protons are electrically charged particles with opposite charges of equal magnitude, between which electric forces act. In addition to protons, we also have neutrons in the nucleus. Neutrons are electrically neutral particles with roughly the same mass as a proton. They contribute to the mass of the element but do not affect the chemical properties of the element.
The mass of an element is mainly in the mass of the nucleus - in protons and neutrons (we ignore the mass of electrons for now). The same element can have different numbers of neutrons in the nucleus; the same atom with a different number of neutrons is called an isotope of an element. Isotopes are the same element but have different masses.
Isotopes of an element are elements that differ from each other only in the number of neutrons (and thus in mass). Since they have the same number of protons in the nucleus (and thus electrons around the nucleus), they are located in the same place in the periodic table of elements.
For practical reasons, it makes sense not to measure the masses of all elements, but to compare them with a unit called the atomic mass unit 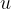 .
.
The scientific community has agreed that 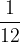 of the mass of the carbon isotope
of the mass of the carbon isotope 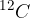 (which is approximately equal to the mass of a hydrogen atom) is chosen as the atomic mass unit
(which is approximately equal to the mass of a hydrogen atom) is chosen as the atomic mass unit 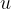 and this is given as:
and this is given as:
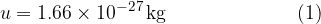
Let the mass of an atom or molecule be denoted by 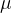 . The relative atomic mass (or mass number)
. The relative atomic mass (or mass number) 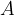 tells how many times the mass of an element (atom or molecule) is greater than the atomic unit of mass
tells how many times the mass of an element (atom or molecule) is greater than the atomic unit of mass 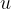 :
:

If 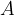 is given as an integer, it means the relative mass of a particular isotope of the element. Since different isotopes of the same element occur in nature, the average relative mass of the isotopes in nature is given in the periodic table. The average relative mass of carbon isotopes is 12.011, and that of the other elements can be found in the periodic table of elements.
is given as an integer, it means the relative mass of a particular isotope of the element. Since different isotopes of the same element occur in nature, the average relative mass of the isotopes in nature is given in the periodic table. The average relative mass of carbon isotopes is 12.011, and that of the other elements can be found in the periodic table of elements.
The mass 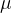 of an atom is given as:
of an atom is given as:
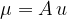
where 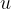 is the atomic unit of mass which is given as:
is the atomic unit of mass which is given as:
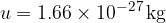
 is the relative atomic mass.
is the relative atomic mass.
Molecules are made up of atoms. The relative molecular mass 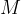 of a molecule in terms of the atomic mass unit
of a molecule in terms of the atomic mass unit 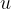 is obtained by adding up all the relative atomic masses
is obtained by adding up all the relative atomic masses 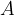 of all the atoms in the molecule:
of all the atoms in the molecule:
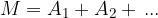
The mass of a molecule is obtained by multiplying the relative molecular mass 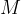 of the molecule by the atomic mass unit
of the molecule by the atomic mass unit 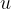 :
:

The mass 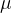 of a molecule is calculated using the formula:
of a molecule is calculated using the formula:
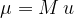
where 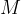 is the relative molecular mass and
is the relative molecular mass and 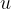 is the atomic unit of mass which is given as:
is the atomic unit of mass which is given as:
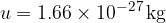
1 kilomole ( ) of a substance is the amount of that substance that has a mass equal to the relative atomic mass
) of a substance is the amount of that substance that has a mass equal to the relative atomic mass 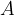 in the case of atoms or relative molecular mass
in the case of atoms or relative molecular mass 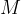 in the case of molecules. So:
in the case of molecules. So:
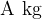 is the mass (or kilomolar mass) of a kilomole of a substance if the substance is made up of individual atoms.
is the mass (or kilomolar mass) of a kilomole of a substance if the substance is made up of individual atoms.
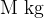 is the mass (or kilomolar mass) of a kilomole of molecules.
is the mass (or kilomolar mass) of a kilomole of molecules.
Another important fact is that there is always the same number of molecules in a kilomole of any substance. This number is called Avogadro's number.
Let's calculate Avogadro's number.
The number 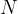 of molecules is the kilomolar mass
of molecules is the kilomolar mass 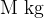 divided by the mass
divided by the mass 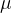 of one molecule:
of one molecule:
We have obtained Avogadro's number, so we add the index 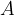 (Avogadro) to the notation
(Avogadro) to the notation 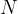 .
.
A kilomole is a unit of mass of a substance. It can be described in two ways:
A kilomole tells us the amount of substance we have, or rather, how many particles (atoms or molecules) there are in a substance. In one kilomole we have exactly Avogadro's number of particles of (any) substance.
A kilomole of a substance also tells us that in one kilomole, there are exactly as many kilograms of a given substance as its relative molecular mass.
The number of particles (atoms or molecules) in a kilomole of (any) substance is always the same and is called Avogadro's number. Avogadro's number is given as: