Personal collections


When electrons move between electron orbitals in an atom, they receive or emit photons of electromagnetic waves.
The word spectrum can have two meanings in physics:
Byspectrum we usually mean the full range of all wavelengths (or frequencies) of electromagnetic radiation.
Sometimes, however, the word spectrum refers to all the wavelengths of waves emitted or received by a certain object:
The emission spectrum of a given substance is the full range of wavelengths of electromagnetic waves emitted by that substance.
The absorption spectrum of a substance is obtained by passing light of all wavelengths through the substance and determining which of these were absorbed or redirected by the atoms of the substance.
The emission or absorption spectrum of a lamp tells us the nature of the light emitted by the lamp. It helps to answer questions such as:
Does the lamp emit photons of all wavelengths or only some?
Does it emit the same number of photons at each wavelength?
At what wavelength does the lamp emits the brightest light?
What is the internal structure of a substance that emits light?
But before we take a closer look at both spectra, let’s first get to know what we call the spectrometer.
A spectrometer is a device used to analyse the spectrum of light.
The spectrometer measures for each individual wavelength how much of this light is incident on it. Simply put, the spectrometer:
counts the photons that fall on its detector,
determines the wavelength of each photon, and
then arrange them by their wavelengths (or frequencies).
In practice, spectrometers typically consist of hundreds of individual detectors and a prism or diffraction grating that disperses and splits the incident light into wavelengths.
If a body is heated or bombarded with particles, it starts emitting light photons, i.e. it starts radiating light. Photons are created when electrons transition from states with higher energy to states with lower energy. The full range of wavelengths that a body emits in this way is called the emission spectrum. Generally speaking, emission spectra are divided into:
continuous
line
Let’s look at both types in detail.
When a body emits light photons of all wavelengths, its emission spectrum is said to be continuous. A continuous spectrum is emitted by more chemically complex substances in which the electron energy levels are so numerous and energetically so close together that they are practically continuous. As a result, photons with arbitrary energies can be produced during transitions between different levels.
Emission spectra of individual atoms and substances in gaseous states consist of individual lines, which are called spectral lines. A spectral line represents a narrow region with a wavelength corresponding to the energy of transitions between different states in atoms. Such a spectrum is called a line spectrum.
The absorption spectrum of a substance is obtained when:
a substance is irradiated with white light containing all wavelengths, and
then the transmitted light by the atoms of the substance is observed with a spectrometer.
Dark lines appear in the spectrum at the wavelengths corresponding to the transition energies of atoms in the substance.
Dark lines appear because:
certain photons of white light are absorbed by the atoms of the substance (these are exactly those photons that have energies corresponding to the wavelength of dark lines),
some electrons are raised to higher levels, and
then again, as the electrons return to their initial state, photons are emitted - but this time, evenly in all directions.
Because of this, in the direction in which the photons travelled at the beginning, the number of absorbed photons is significantly reduced and therefore the light flux density for these photons is also much lower.
X-rays are high-energy electromagnetic waves. The wavelengths of X-rays range between 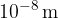 and
and 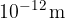 - which is much more than e.g. visible light, which has wavelengths around
- which is much more than e.g. visible light, which has wavelengths around 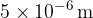 .
.
Photons of X-ray light have energies between 0.1 keV and 1 MeV and are produced when very fast charged particles (e.g. electrons, protons,...) stop in matter. A possible source of X-ray radiation is also electron transitions between levels with very different energies in heavy atoms.
In X-ray machines or X-ray tubes, X-rays are created using the first principle - that is, by stopping fast particles in matter, as shown in Figure 6:
Let's describe what happens in Figure 6:
Electrons are first thermally emitted from the cathode;
Then, they are accelerated to high speed by the electric field between the cathode and the anode.
The electrons then collide with the anode, where they also stop and emit X-ray photons (as well as the entire spectrum of photons of lower energies).
Such continuous radiation is called bremsstrahlung radiation since photons are produced when an electron is "braked or stopped" by matter.
The maximum energy 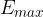 that a photon generated in an X-ray tube can have is determined by the voltage
that a photon generated in an X-ray tube can have is determined by the voltage 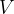 between the anode and the cathode and is equal to the energy received by the electron during acceleration:
between the anode and the cathode and is equal to the energy received by the electron during acceleration:
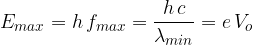
An electron can stop in a substance in the following ways:
it suddenly stops completely and gives all its energy to the emitted photon. In this case, we get a photon with the maximum possible energy, which is equal to the kinetic energy of the incident electron.
it stops in the substance in several steps, which means that it bounces from atom to atom and with each interaction with the atom, gives up a smaller amount of energy and thus emits more photons with lower energies on its way.