Personal collections


Since we want to understand how atoms interact with photons and with each other, we must first explain how electrons are arranged around an atom and how they can receive and emit energy.
Experiments have shown that electrons in an atom occupy electron shells which have well-defined energies. When an electron passes from one shell (state) to another, it releases or consumes energy:
The change in energy 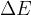 of the electron is given as:
of the electron is given as:
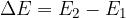
where:
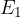 is the energy of the electron in the initial state and
is the energy of the electron in the initial state and
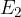 is the energy of the electron in the final state.
is the energy of the electron in the final state.
In the material on the components of an atom, we said that the electron shell is that part of the atom in which the electrons are located. Inside the atom, individual electrons stay in areas called electron shells and each electron has well-defined energy in the shell.
An electron can pass from one shell to another only by emitting or receiving some energy. This usually happens with the help of a photon:
If an electron receives energy from a photon, the photon disappears and the electron enters a higher energy state.
If an electron emits energy by emitting a photon, a photon is formed and the electron enters a lower energy state.
Because electrons have well-defined energies in all shells, well-defined energies are released or emitted during these transitions.
The energies of shells are characteristics of the type of atom or molecule. For example, in an oxygen atom, electrons have different energies and occupy different shells from in a lead atom or a nitrogen molecule.
Figure 1 shows a typical distribution of energies held by electrons in different shells in an atom:
Let's describe Figure 1:
The energy of electrons increases along the ordinate (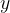 ) axis.
) axis.
The straight horizontal lines in the figure represent specific energies. Bound electrons cannot have energies other than these specified values:
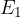 is the energy of the electron that occupies the lowest shell and is most strongly attached to the atom.
is the energy of the electron that occupies the lowest shell and is most strongly attached to the atom.
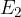 is the energy of the electron in the second lowest shell.
is the energy of the electron in the second lowest shell.
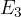 is the electron energy in the third lowest shell.
is the electron energy in the third lowest shell.
etc.
The gray-colored field indicates free electrons. The energy of free electrons can have any value.
By convention, electrons bound in atoms have negative energies. The more strongly they are bound, the lower their energies. The electrons in the shells farther from the nucleus are weaker bound and their binding energy is approaching zero. When an electron has binding energy equal to zero, we speak of a free and stationary electron. The energies of free and moving electrons are positive.
When an electron passes from a shell with energy 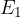 to a shell with energy
to a shell with energy 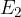 , the energy it receives (or emits when the electron goes in the opposite direction) is equal to:
, the energy it receives (or emits when the electron goes in the opposite direction) is equal to:
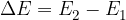
As the Energy Conservation Law applies, this energy can be emitted or received in several different ways, either by collision with another atom or by emission (emission) or absorption (reception) of a photon.
Let's describe the figure above:
the left shows the transition of an electron from a higher energy state to a lower one; in this transition, the electron emits a photon.
the right shows the transition of an electron from a lower energy state to a higher one; at this transition, the electron receives a photon.
In both cases, the energy of the photon is equal to the difference between the energies of the two states.