Personal collections


Briefly describe the concept of a photon.
Explain how lines in the emission spectrum of gases at low pressure provide evidence for discrete electron energy levels in atoms.
Three electron energy levels in atomic hydrogen are represented in the figure below.
The wavelengths of the spectral lines produced by electron transitions between these three energy levels are 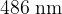 ,
, 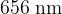 , and
, and 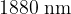 .
.
On the figure above, draw arrows to show the electron transitions between the energy levels that would give rise to these wavelengths. Label each arrow with the wavelength of the emitted photon.
Calculate the maximum change in energy of an electron when making transitions between these levels.