Personal collections


A beam of white light passes through a cloud of cool gas. The spectrum of the transmitted light is viewed and contains a number of dark lines. Explain why these dark lines occur.
Some energy levels for the electron in an isolated hydrogen atom are illustrated in the figure below.
The table below shows the wavelengths of photons that are emitted in the transitions to 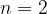 from the other energy levels shown in the figure above.
from the other energy levels shown in the figure above.
The energy associated with the energy level 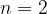 is
is  . Calculate the energy, in
. Calculate the energy, in  , of energy level
, of energy level 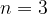 .
.