Personal collections


Explain how a line emission spectrum leads to an understanding of the existence of discrete electron energy levels in atoms.
Some of the lines of the emission spectrum of atomic hydrogen are shown in the figure below.
The photon energies associated with some of these lines are shown in the table below.
Complete the table above by calculating the photon energy for a wavelength of 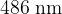 .
.