Personal collections


Explain how the line spectrum of hydrogen provides evidence for the existence of discrete electron energy levels in atoms.
Some electron energy levels in atomic hydrogen are illustrated in the figure below.
Two possible electron transitions, A and B, giving rise to an emission spectrum, are shown. These electron transitions cause light of wavelengths 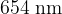 and
and 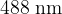 to be emitted.
to be emitted.
On the figure above, draw an arrow to show a third possible transition.
Calculate the wavelength of the emitted light for the transition in (i).