Personal collections


Figure I below shows the lowest four energy levels of an electron in an isolated atom.
Figure II below shows the lines in the emission spectrum of the atom that correspond to the transitions of the electron from 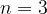 to
to 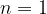 and from
and from 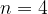 to
to 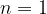 .
.
Explain, with reference to photons, why there is a single frequency of electromagnetic radiation that corresponds to each of these transitions.
On Figure II, draw a line that corresponds to the transition of the electron from 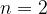 to
to 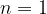 . Label this line A.
. Label this line A.
On Figure II, draw a line that corresponds to the transition of the electron from 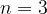 to
to 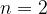 . Label this line B.
. Label this line B.
The frequency of radiation represented by line A is 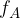 . The frequency of radiation represented by line B is
. The frequency of radiation represented by line B is 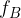 . The energy of the ground state (
. The energy of the ground state (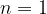 ) is
) is 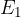 . Determine an expression, in terms of
. Determine an expression, in terms of 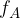 ,
, 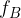 ,
, 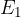 and the Planck constant
and the Planck constant 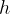 , for the energy
, for the energy 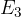 of the energy level
of the energy level 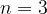 .
.