Personal collections


White light is incident on a cloud of cool hydrogen gas, as illustrated in Figure I below.
The spectrum of the light emerging from the gas cloud is found to contain a number of dark lines.
Explain why these dark lines occur.
Some electron energy levels in a hydrogen atom are illustrated in Figure II below.
One dark line is observed at a wavelength of 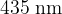 .
.
Calculate the energy, in 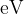 , of a photon of light of wavelength
, of a photon of light of wavelength 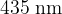 .
.
On figure II above, draw an arrow to indicate the energy change that gives rise to this dark line.