Personal collections


The sodium atom has an ionization energy of 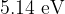 . This is the energy needed to knock an outer electron in the ground state out of the sodium atom. Sodium ion
. This is the energy needed to knock an outer electron in the ground state out of the sodium atom. Sodium ion 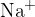 is obtained in the transition of an outer electron from the first excited state to the ground state alongside light with a wavelength of
is obtained in the transition of an outer electron from the first excited state to the ground state alongside light with a wavelength of 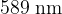 . What kind of light do we need to ionize a sodium atom that has an outer electron in the first excited state?
. What kind of light do we need to ionize a sodium atom that has an outer electron in the first excited state?