Personal collections


The specific latent heat of vaporisation of water at atmospheric pressure of 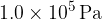 is
is 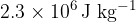 . A mass of
. A mass of 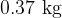 of liquid water at
of liquid water at 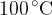 . is provided with the thermal energy needed to vaporise all of the water at atmospheric pressure.
. is provided with the thermal energy needed to vaporise all of the water at atmospheric pressure.
Calculate the thermal energy 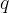 supplied to the water.
supplied to the water.
The mass of  of water is
of water is 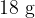 . Assume that water vapour can be considered to behave as an ideal gas. Show that the volume of water vapour produced is
. Assume that water vapour can be considered to behave as an ideal gas. Show that the volume of water vapour produced is 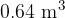 .
.
Assume that the initial volume of the liquid water is negligible compared with the volume of water vapour produced. Determine the magnitude of the work done by the water in expanding against the atmosphere when it vaporises.
Use your answers in (a)(i) and (a)(iii) to determine the increase in internal energy of the water when it vaporises at 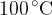 . Explain your reasoning.
. Explain your reasoning.
Use the first law of thermodynamics to suggest, with a reason, how the specific latent heat of vaporisation of water at a pressure greater than atmospheric pressure compares with its value at atmospheric pressure.