Personal collections


An ideal gas has amount of substance 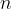 . The gas is initially in state X, with pressure
. The gas is initially in state X, with pressure 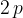 and volume
and volume 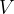 . The gas is cooled at constant volume to state Y, with pressure
. The gas is cooled at constant volume to state Y, with pressure 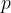 . The gas is then heated at constant pressure to state Z, with volume
. The gas is then heated at constant pressure to state Z, with volume 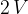 . Finally, the gas returns at constant temperature to state X.
. Finally, the gas returns at constant temperature to state X.
During the change of state from Y to Z, the increase in internal energy of the gas is 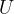 . During the change of state from Z to X, the work done on the gas is
. During the change of state from Z to X, the work done on the gas is 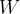 . Complete the table below to indicate, for each of the three changes of state, the increase in internal energy of the gas, the thermal energy transferred to the gas and the work done on the gas, in terms of
. Complete the table below to indicate, for each of the three changes of state, the increase in internal energy of the gas, the thermal energy transferred to the gas and the work done on the gas, in terms of 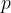 ,
, 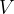 ,
, 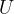 and
and 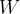 .
.